Chromogenic detection of xylene isomers and luminogenic chemosensing of o-xylene employing a new macrocyclic cobalt complex: synthesis, and X-ray crystallographic, spectroscopic and computational studies†
Abstract
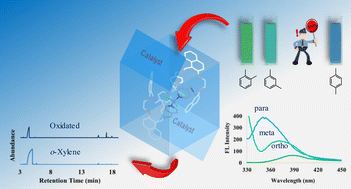
Here, we report the synthesis and characterization of a binuclear Co(II) complex (Co2(2py)2Cl4) with two dinaphtho-diazacrown ether macrocyclic ligands, bearing two pyridine arms as a colourimetric and fluorescent sensor for detecting different xylene isomers as well as acting as a catalyst for the oxidation of o- and m-xylene under vacuum at room temperature. Chromogenic detection occurred when Co2(2py)2Cl4 was exposed to the xylene isomers, wherein the original blue colour of the complex changed to green and green-blue in the presence of o- and m-xylene, respectively. Meanwhile, no colour change was observed in the presence of the p-xylene isomer. Fluorescence spectroscopy revealed that Co2(2py)2Cl4 is a luminogenic chemosensor for o-xylene. The maximum fluorescence quenching occurred in the presence of o-xylene while the least interaction with p-xylene was observed. Different quenching of the FL emission band of Co2(2py)2Cl4 in the presence of p-xylene implied different interactions of the complex, compared to the other two isomers. From FT-IR spectroscopic, 1H NMR, and TGA studies, it has been observed that Co2(2py)2Cl4 binds o-xylene more strongly than bindng the other two isomers. GC-MS analysis of the filtered o-xylene as the contact solvent with the dispersed Co2(2py)2Cl4 indicated the catalytic formation of some products assignable to 2-methyl-benzaldehyde, 1-methoxymethyl-2-methylbenzene, 2-methyl-benzoic acid, and 2-methyl-benzenemethanol, while no detectable corresponding product was found for the other two xylene isomers. Density functional theory calculations indicate that the o-xylene dissolution supports the structure to overcome the intramolecular forces and provides the enquired space (∼12 Å) for a xylene molecule to approach the cobalt. The optimized structure of the xylene-Co2(2py)2Cl4 inclusion species based on its optimized geometry in DMF showed that o- and m-xylene yielded 12.45 and 18.53 kcal mol−1 energy differences, respectively. Time-dependent DFT calculations (PBE0-TDDFT) for Co2(2py)2Cl4 electronic transitions are also reported.


 Please wait while we load your content...
Please wait while we load your content...